Mood: impish
Yesterday I attended the Washington Wildlife Rehabilitation Association's Fifth Annual Washington Wildlife Rehabilitation Conference. While not an wildlife rehabber myself, I am very interested in the community--they have a wealth of good experience and knowledge, and they are definitely on the side of the angels in their work to help wildlife. I want to direct a significant part of my future anatomy informatics research toward developing tools for them to be able to share, organize, and find that information as seamlessly as possible.
The program was crammed full of good information, and I have a lot of biomedical and behavioral information to process into usable form, and many new contacts to follow up on. They ended on a fun and useful note, with a workshop on raptor imping, or feather repair for Washington birds of prey, such as hawks and owls. It's kind of like a hair transplant for avians, but it's much more practical than cosmetic--it can make a lot of difference in flight stability, which itself can be a decisive factor in deciding whether or not a bird is ready for re-release into the wild.
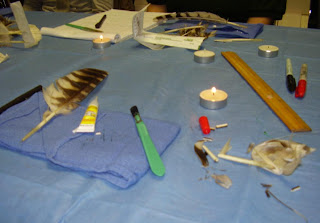
I hasten to reassure Washington hawks, owls, pelagic birds, and others that I have no intention of actually practicing on real birds any time in the near future! For me, it was an opportunity to learn some non-mammalian anatomy, and--more important--to get a hands-on opportunity to see how wildlife rehabbers gain, process, and share new information with applied (clinical) relevance, in order to incorporate those observations into some future way of information-sharing. I appreciate Mike's (the instructor) taking his time and materials to provide the instruction and practice, and WWRA for providing the venue and opportunity to take the workshop.

In real life, of course, you'd be carrying out this procedure on a live bird in rehabilitation; we practiced on isolated feathers instead. As good an idea as that sounds in theory, you'll also see its wisdom borne out in practice as the workshop unfolded. Below, in the bottom right quadrant of the photo, you see my broken feather (a left #10 primary from a barred owl, to be precise) on the left, and a replacement left #10 primary feather from a different barred owl on the right.

By the time I had taken this photo, I had already separated the calamus of the broken feather (left) from its vane (center). If this were a real-life situation, the calamus would still be attached in the follicle of the feather in the bird, who would be restrained or anesthetized, lying there while the procedure is carried out. The replacement feather (right) has not yet had the calamus separated from the vane, which will be the next step.
That's where the tea candles you see in the picture come in--if you take the time to heat the scalpel blades thoroughly in the flame, then there's no slicing or sawing through the shaft. The warm blade cuts through the quill like butter.
Fortunately, Mike stepped us through it patiently, because at a conceptual level, I was not sure I understood what had to happen to what in what order. There seemed to be a similar concern elsewhere in the room--I heard people doubting whether they could do this. After all, the animal's welfare is the primary concern, and they (and I) do not want to screw that up. But the people in the room, other than me, carry out other difficult procedures to help injured and recovering wildlife every day, and I had no doubt that the rehabbers who want to go on in imping will do so successfully, after some practice.
Below the calamus has been separated from the vane in both feathers, and in the left of the field, the calamus of the broken feather (still embedded in the follicle of the live bird, remember!) is roughly lined up with the vane of the replacement feather. Notice how I've been moving the calamus around in the photos to get a comfortable alignment for me--in real life, that poor bird would not need to be rotated so much and so often; part of this skill, I think, must be learning where things need to be, and setting them up that way at the start.
The calamus of the replacement feather and the vane of the broken feather are at right, and can be discarded, except I am going to try to use the shaft of the broken feather as the bridge for my imping.

Success! Or, at least, half-success; I still have to do the same thing for the calamus.
The bridge slides in to the shaft of the vane, and fits snugly--there is no looseness or rattling around, which will have an adverse effect on the success of the imping.
At this point, I am feeling rather pleased with myself, although I am concerned about how long it is taking me, with my "patient" either restrained or under anesthetic. Birds, after all, stress out very easily; I think that developing skillful speed must be an important component of this.
Other people in the room are having similar concerns; from somewhere, I hear the following exchange:
"Oh, no; we took too long--the patient just died!"
"Well, check and see whether he's a feather donor."
My bridge to the calamus was not quite as successful as to the vane; it was a little floppy. But the workshop was winding up, so I didn't have time to start a whole new bridge. For the sake of practice, I went with this one, and now that the bridges were in place, it was time to bind them permanently.
We had had a discussion at the beginning of properties of glue; epoxy actually seems to be the best choice, although sea water can cause some kinds to lose their integrity. The choice of glue is a non-trivial question, in other words, but for the sake of a practice workshop, Mike chose SuperGlue because it's cheap and fast.

I already had a certain amount of doubt about the safety of birds, blades, and flames around my motor skills; accidentally SuperGluing a bird's wing together seemed to me like a real risk to be avoided, and I promptly proved the point by SuperGluing my right ring finger and pinky into kind of a mirror-image "OK" sign (or "Arschloch" sign, if you're a German-speaker.) Ironically, if I were a pterosaur, I would have just totally subverted the entire imping procedure, since while bats fly on their fingers, and birds fly on their arms, in pterosaurs, "the ring finger is the wing finger" (I'll attribute that quote if I can find where I heard it), so I had just taken out my entire wing.
"Help", I said softly to Mike, who sized up the situation, pulled my hand up over my head like the referee does a boxing champ, and announced, "Can I have everyone's attention, please? Don't do this." (waving my hand). Fortunately, one of the rehabbers was also an experienced mom, who has obviously lived the SuperGluing fingers together scenario before. With warm water, a pen to gradually work the fingers apart, and a minimum of drama, we got it under control.

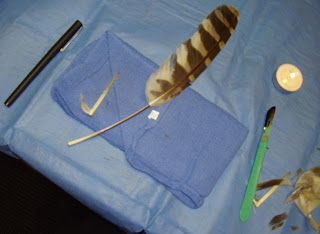
My results, while not perfect, were actually not bad for a first attempt, although as said above, it took a very long time. Below are pictures of my imped feather.


I did not get to keep my imped feather, as this is an area where Federal law, tribal law, endangered species, and freedom of religion all intersect, sometimes quite contentiously, and there is a lot of paperwork involved to make sure that everyone is cleaner than Caesar's wife about the use of raptor feathers. Mike and WWRA have the required permits, and can put on the workshop, but to transfer the feathers to us, who do not all have those permits, is a ton of effort, accounting, and documentation that we did not go to.
I totally had a blast, I learned something about avian anatomy, and more about the rehab community and how they gain, share, and use clinical information. All in all, a day very well-spent.
These are very good people, and they do important work to help animals. I invite you to support your local wildlife rehabber, as well as local and national wildlife rehab organizations: the National Wildlife Rehabilitators Association and the International Wildlife Rehabilitation Council. Locally, I am going to donate my time and money to WWRA, and I invite my readers (both of you! :) to do likewise.
Labels: anatomy informatics, animals, crafts, endangered species, knowledge representation, people-watching
Read more!














